Who Invented Batteries?
Most of us use batteries in many shapes and forms in our everyday lives and take them for granted. From the alarm clock that wakes us up in the morning and the car that we drive to work, to the pacemaker that keeps us alive and phone battery that lets us communicate. But batteries have gone through a long journey of discovery and development to get to where they are today.
In this listicle, we take a look at some of the landmark advancements in the history of batteries that have helped to shape their improvement and evolution (Figure 1).
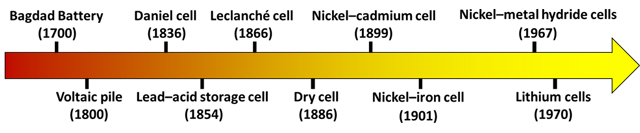 Figure 1: A timeline indicating the major advancements in battery development. Credit: The Author.
Figure 1: A timeline indicating the major advancements in battery development. Credit: The Author.
Bagdad Battery (1700): In 1938, Wilhelm Konig, a German archaeologist, unearthed earthenware jars of approximately the size of a human fist at Khujut Rabu, located near Bagdad, Iraq. These 2,200-year-old jars were comprised of an iron rod within a copper cylinder, sealed with an asphalt stopper. It is speculated that these jars were utilized by the inhabitants of the Parthian civilization, which governed the region 2,000 years ago, as electrical batteries for electroplating gold onto silver. This assemblage has become known as the “Bagdad Battery”. However, it is important to note that there is currently no concrete evidence supporting this speculation, and even the dating of these artifacts remains somewhat disputed.1
Voltaic pile (1800): Allesandro Volta, who is considered to be the “real” discoverer of batteries, 1 made and introduced the first successful demonstration of a modern battery, commonly referred to as the Voltaic pile. This device consisted of a series of zinc and silver plates stacked together, with each plate separated by a cloth soaked in a solution of acid and salt. This invention marked a significant milestone, as it paved the way for revolutionary advancements in long-distance communication, including the development of telegraphs in the late 1830s and, much later, the telephone in the 1870s. However, the original Voltaic pile encountered a challenge due to the development of hydrogen bubbles as a result of chemical reactions that adhered to the electrode surfaces. This issue led to a rapid decline in the performance of the battery, rendering it of limited practical use.
Daniel cell (1836): English chemist, John Daniel solved the performance degradation of the Voltaic pile in 1836 with the discovery of a two-fluid battery, named a Daniel cell.2 The system, consisted of a glass jar with a zinc anode on top and a copper cathode at the bottom. A two-layered liquid of concentrated CuSO4 and dilute H2SO4 was used as the electrolyte. It was commercially exploited mostly for powering telegraphs until the late 19th century when the introduction of other novel designs overshadowed its prominence. All of the various designs of batteries were until now based on a single use philosophy, where the electrodes, once consumed by the chemical reaction, could not be regenerated, defining what are now called primary cells.
Lead–acid storage cell (1854): German physicist Wilhelm Josef Sinsteden in 1854, brought to light the concept of rechargeable batteries by utilizing two lead sheets in a container of dilute H2SO4. Soon after, in 1859, French physicist, Gaston Planté introduced the first rechargeable lead–acid battery3 that revolutionized the world. It consisted of a dual sheet of lead with a rubber strip between them as a separator, which was again rolled into a spiral and immersed in dilute H2SO4 electrolyte.
Leclanché cell (1866): In 1866, George Leclanché, a French physicist, introduced several significant innovations and deviations from the prevailing approach of that time. He developed a new type of battery that utilized MnO2 as one of the electrodes, marking the first use of an oxide for this purpose.1 Lead oxide was not incorporated into the design of lead–acid batteries until 1881. The telegraphic service of Belgium swiftly adopted this technology in 1867, showcasing an exceptionally rapid transition from patent to market.1 Leclanché also introduced the use of NH4Cl solution as the electrolyte,4 which diverged from the prevailing practice of employing protonic acids.
Dry cell (1886): Carl Gassner, in 1886, replaced the liquid NH4Cl solution in the Leclanché cell with a paste of NH4Cl solution mixed with plaster of Paris, creating the first significant dry cell.5 Gassner's invention was patented in multiple countries because he knew the commercial potential of his innovation. During the same period, there were also independent developments of dry batteries, including notable contributions by Wilhelm Hellesen and Yai Sakizo. The precise attribution of who invented the first dry cell remains somewhat unclear.
Nickel–cadmium cell (1899): W. Jüngner introduced the nickel–cadmium battery in 1899, which was the first alkaline battery.6 It quickly became renowned as the ideal battery technology for small consumer devices. This technology was praised for its high current capacity and the ability to undergo numerous charging-discharging cycles. However, in the 1930s, it began to lose popularity due to factors like its high cost, degradation of the electrolyte, reduced battery lifespan and the toxic nature of cadmium.
Nickel–iron cell (1901): In addition to his work on nickel–cadmium batteries, Jüngner also introduced nickel–iron batteries. This design was further developed and patented by Thomas Edison in 1901. It is intriguing to observe that Edison's motivation for enhancing nickel–iron batteries stemmed from his ambition to make electric cars the preferred means of transportation, a sentiment that is experiencing a resurgence over a century later. Unfortunately, the issue of iron poisoning posed a significant obstacle to the commercial viability of these batteries, leading to their decline in popularity.
Nickel–metal hydride cells (1967): The unpopularity of cadmium encouraged the development of nickel−metal hydride batteries in 1967, which are not only cadmium-free but can store more charge than nickel–cadmium batteries. On the downside, nickel−metal hydride batteries deliver less power, have a faster self-discharge and are less tolerant to overcharge.1 They found wide applications in mobile phones, computers and portable electronic goods after their commercialization in 1991.
Lithium cells (1970): Lithium-based batteries were the last to emerge in the progression of battery technology, only introduced in the 1970s by various research groups. The concept of lithium-ion (Li–ion) batteries was initially discussed around 1979 after which significant advancements were made, starting in 1980 with N. A. Godshall and colleagues,7 as well as J. B. Goodenough and colleagues.8 Their works successfully demonstrated the insertion and extraction of a lithium ion in LiCoO2, along with other oxide systems. These breakthroughs laid the foundation for the commercialization of Li–ion battery technology, which was achieved by Sony in 1991.9 Li–ion batteries found widespread use in pacemakers, watches, cameras and portable electronic devices, as well as electric and hybrid vehicles. Both rechargeable10 and non-rechargeable variants are available. Currently, scientists are actively exploring alternative battery technologies, such as sodium–ion,11 lithium–air and lithium–sulfur batteries, which hold great promise but also present their own set of challenges.12
Battery development and capabilities have come a long way over the years, but this field is continuing to evolve at a rapid pace. Scientists are striving for batteries with enhanced capabilities, improved safety and a more environmentally friendly footprint to meet consumer and policy demands. Consequently, we are likely to see more exciting additions to this timeline in the years to come.
1. Sarma DD, Shukla AK. Building better batteries: A travel back in time. ACS Energy Lett. 2018;3(11):2841-2845. doi:10.1021/acsenergylett.8b01966
2. Daniell JF. On voltaic combinations. In a letter addressed to Michael Faraday, d. C. L., f. R. S., fullerian prof. Chem. Royal institution, corr. Memb. Royal & imp. Acadd. Of science, Paris, petersburgh, &c. By j. Frederic daniell, f. R. S., prof. Chem. In King's College, London. Phil Trans R Soc. 1836;126:107-124. doi:10.1098/rstl.1836.0012
3. Kurzweil P. Gaston planté and his invention of the lead–acid battery—the genesis of the first practical rechargeable battery. J Power Sources. 2010;195(14):4424-4434. doi:10.1016/j.jpowsour.2009.12.126
4. Dutta A, Mitra S, Basak M, Banerjee T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage. 2023;5(1). doi:10.1002/est2.339
5. Mertens J. The development of the dry battery: Prelude to a mass consumption article (1882-1908). Centaurus. 2000;42(2):109-134. doi:10.1034/j.1600-0498.2000.420203.x
6. Pourabdollah K. Development of electrolyte inhibitors in nickel cadmium batteries. Chem Eng Sci. 2017;160:304-312. doi:10.1016/j.ces.2016.11.038
7. Godshall NA, Raistrick ID, Huggins RA. Thermodynamic investigations of ternary lithium-transition metal-oxygen cathode materials. Mater Res Bull. 1980;15(5):561-570. doi:10.1016/0025-5408(80)90135-X
8. Mizushima K, Jones PC, Wiseman PJ, Goodenough JB. Lixcoo2 (0<x<-1): A new cathode material for batteries of high energy density. Mater Res Bull. 1980;15(6):783-789. doi:10.1016/0025-5408(80)90012-4
9. Manthiram A. An outlook on lithium ion battery technology. ACS Cent Sci. 2017;3(10):1063-1069. doi:10.1021/acscentsci.7b00288
10. Goodenough JB, Kim Y. Challenges for rechargeable li batteries. Chem Mater. 2010;22(3):587-603. doi:10.1021/cm901452z
11. Luo W, Shen F, Bommier C, Zhu H, Ji X, Hu L. Na-ion battery anodes: Materials and electrochemistry. Acc Chem Res. 2016;49(2):231-240. doi:10.1021/acs.accounts.5b00482
12. Duffner F, Kronemeyer N, Tübke J, Leker J, Winter M, Schmuch R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat Energy. 2021;6(2):123-134. doi:10.1038/s41560-020-00748-8