Boost the Boundaries of Early Drug Discovery
For small-molecule drugs and biologics alike, the path to a marketed drug involves a long and exhaustive journey through basic research, discovery of the medicine, preclinical development tests, increasingly complicated clinical trials, and finally the regulatory approval.
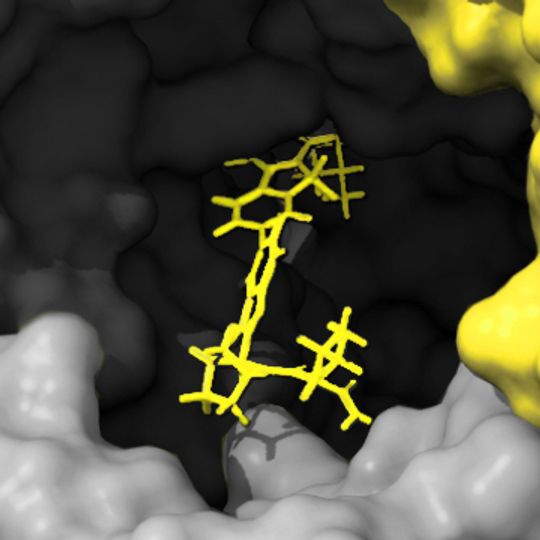
Fragment-based drug design (FBDD) has become a popular platform for the identification of lead candidates in drug discovery programs alongside structure-based drug design, and high throughput screening. In 2021 over 70% of the drugs approved by the FDA were non-protein based and therefore the ability to measure the kinetics & affinity across a range of molecular sizes is critical during initial selection.
The detection and characterization of these binding events is facilitated by sensitive biophysical technologies capable of detecting low affinity interactions of low molecular weight compounds. Surface plasmon resonance (SPR) is a core technology in many pharma and biotechnology settings for this purpose as SPR has the required sensitivity and throughput to provide complete fragment screens on libraries of several thousand compounds in just a few weeks per target.
Traditional initial SPR screening of weak affinity compounds using a single fixed concentration injection can present numerous challenges, which include low molecular weights, weak affinities (μM – mM), and solubility limitations that make it impractical to test for binding using sample concentrations above the KD. When combined this can frequently result in the need to make decisions based on inappropriate (small, square-shaped) sensorgrams.
Due to these restrictions, there is a need for techniques that can resolve these limitations and increase hit confidence, shorten timelines, and allow screening groups to provide richer datasets for more comprehensive analysis.
OneStep® Injections, which are unique to the Octet® SF3 SPR, use a continuous analyte titration method and can dramatically improve SPR-based screening by providing higher content information that allows for confident, rapid characterization of hits. The single gradient injections allow users to obtain accurate kinetic rate constants (ka, kd), affinity (KD) and ligand efficiency (LE) data directly from the primary screen, and as such combine the first three steps (clean screen, primary yes/no screen, kinetics and affinity hit confirmation) in the traditional SPR workflow into a single assay.
Here, using a well-characterized small molecule interaction system, we demonstrate that standard multi-cycle kinetics (MCK) do not provide sufficient data from a single concentration injection, and that reliable kinetics and affinity can be obtained from significantly less data than is commonly used. In addition, the number of measurements necessary for accurate kinetics and affinity determination can be reduced to a single measurement, thus increasing sample throughput and saving sample material.
This removes the barrier for SPR as a tool for large screens and allows rapid progression for additional assay development of potential therapeutics.
Revolutionize Your FBDD with the next generation of SPR systems and discover how to:
- Save time, effort, and samples
- Obtain accurate kinetics parameters from your primary screen
- Identify drug candidates confidently from day one